Study of Mechanisms of Ion Transport in Ion Conducting Glasses
P. Bury a), P. Hockicko a), M. Jamnický b)
and I. Jamnický a)
a) Department of Physics, Žilina University, 010 26 Žilina, Slovakia (bury@fel.utc.sk)
b) Department of Ceramic, Glass and Cement, Slovak
Technical University, 812 37 Bratislava
Abstract In the past twenty years, there has been an increase of
interest in ion conductive glasses and their possible future practical
application. Comparing with the crystalline materials the ionic glasses has
several advantages the most important of which are: the absence of grain
boundaries, the isotropic properties and the composition variability. The
electrical methods have been already proved an effective tool to study the
fundamental transport properties of the ionic materials and the conductivity
measurements made over a wide range of frequencies and temperatures can
characterise different types of transport mechanisms. The purpose of present
work is to compare data obtained by electrical measurement (dc and ac
conductivity) of ion conductive glasses of the system CuI-CuBr-Cu2O-(P2O5+M0O3)
for different glass composition and study the ionic hopping motion and
relaxation processes connected with the charge mobility. Partial attention is
paid also to the problem of the role of composition in this system.
I. Introduction A considerable
attention is given in recent years to glassy materials with the fast ion
transport called solid state electrolytes because of the possibility of their
application in modern electrochemical devices, such as solid-state batteries,
electrochronic displays, and sensors as well as for fundamental interest in
their ion transport mechanisms [1,2]. The ion conductive glasses have several
advantages comparing with crystalline materials because of their easy
preparation, their stability and the large available composition ranges.
The
investigation of conductivity spectra of ionic glasses can reflects the basic
features of the relaxation and transport mechanisms of the mobile ions and the
high ion conductivity at room temperature is the most important criterion witch
should be meet the fast ion conductive glasses. Here is a good opportunity for
glasses containing Cu+ conductive ions that have similar electronic
configuration and smaller ionic radii in comparison with Ag+ ion and
could achieve comparable conductivity [3].
While
there are many papers on Ag+-ion conducting glasses in various
glass-forming systems, Cu+-ion conducting glasses are only known in
very limited glass-forming systems.
Phosphate glasses containing Cu+ conducting ions are good ionic conductors
with room temperature conductivity of the order 10-3W-1cm-1
[4,5]. The highest conductivity has been recorded in systems containing large
more fractions of cuprous halides, such as CuI or CuBr. Moreover, if two
different kinds of halide anions are mixed into cation conducting glasses [6],
a positive deviation of the electrical conductivity from the additivity rule
can be observed (mixed anion effect). In this contribution we present some
electrical (transport) properties of glasses prepared in the
systems CuI-CuBr-Cu2O-MmOn where MmOn
is P2O5 and/or MoO3. The main purpose of the
contribution is to investigate ion transport mechanisms and to determine the
relation between glass composition and electrical conductivity.
II. Experimental The preparation
of glasses in the system CuI-CuBr-Cu2O-(P2O5+MoO3)
from commercial reagents (Fluka) represented the procedure already described
[6]. Batches of 15 g were melted under a dry argon atmosphere to avoid the
oxidation of Cu+ during melting and mixed in appropriate portion (see Tables 1 and 2) in silica ampoule at 933 K for 90 min. The
glass melts were rapidly quenched by pressing them between two brass plates to
a final thickness of » 1.5 mm. The resulting discs of 20 mm in diameter were kept between the
plates until their temperature decreased to room temperature. Losses in weight
during melting were < 1 %. To check the reproducibility of the results, all glasses were
prepared three times. Two systems of glasses were prepared to investigate both
the role of glass-forming system and the role of cuprous halides produced Cu+
ions keeping their ratio 60 mol. % to 40 mol. %.
The
samples for electrical conductivity measurements were cylindrical in shape
(area » 1 cm2, thickness » 1-2 mm). Gold electrodes were sputtered onto the
sample surfaces. The frequency and temperature dependencies of electrical
conductivity were measured (d.c. and a.c. in the frequency range from 50 Hz up
to 1 MHz using FLUKE PM 6306 impedance analyser and in the temperature range of
140-380 K temperature range ~ 100°C. The measured complex impedance allowed us to
obtain the bulk d.c. and a.c. conductivity of glass samples by means of the
usual impedance analysis.
Table 1 Starting compositions (in
mol.%) of glasses prepared in the System I
|
Glass |
|
Composition |
(in mol.%) |
|
|
|
sample |
CuI |
CuBr |
Cu2O |
P2O5 |
MoO3 |
|
IP |
25.000 |
- |
56.250 |
18.750 |
- |
|
IPM |
25.000 |
- |
46.875 |
9.375 |
18.750 |
|
BPM |
- |
25.000 |
46.875 |
9.375 |
18.750 |
|
IM |
25.000 |
- |
37.500 |
- |
37.500 |
Table 2 Starting compositions (in
mol.%) of glasses prepared in the System II
|
Glass |
|
Composition |
(in mol.%) |
|
|
|
sample |
CuI |
CuBr |
Cu2O |
P2O5 |
MoO3 |
|
IBPM 2 |
21.875 |
3.125 |
46.875 |
9.375 |
18.750 |
|
IBPM 3 |
18.750 |
6.250 |
46.875 |
9.375 |
18.750 |
|
IBPM 5 |
15.625 |
9.375 |
46.875 |
9.375 |
18.750 |
|
IBPM 1 |
12.500 |
12.500 |
46.875 |
9.375 |
18.750 |
|
IBPM 4 |
6.250 |
18.750 |
46.875 |
9.375 |
18.750 |
![]()
![]()
III.
Results The measured complex impedance
allowed to obtain the bulk d.c. and a.c. conductivity of glass samples of both
systems at given temperature range.
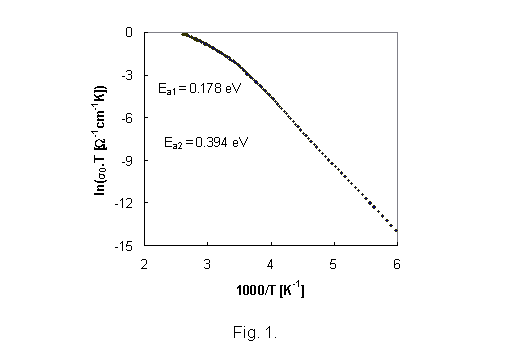
The
representative results of d.c. conductivity measurement (sample IBPM 2) as a
function of temperature are illustrated in Fig. 1. As all of the temperature
dependence of d.c. glass conductivity can be fitted by the equation
s = s0 exp(-Ea/kT), (1)
where Ea is the activation energy, k is the Boltzman constant and T is the absolute temperature, the temperature dependencies of d.c.
conductivity indicates two transport mechanisms with activation energies Ea1, and Ea2 for higher and lower temperatures, respectively.
Because the pre-exponential factor s0 is a function of temperature the factor sT is used in Arrhenius
plots of d.c. conductivity. Activation energies calculated from Arhenius plots
of d.c. conductivity for all glass samples are summarised in Table 3.
Table
3. Summary of activation energies calculated from Arrhenius plots of d.c. conductivity for glasses of both systems
|
Glass sample |
Ea1 (eV) |
Ea2 (eV) |
Glass sample |
Ea1 (eV) |
Ea2 (eV) |
|
IP |
0.295 |
0.500 |
IBPM 2 |
0.178 |
0.394 |
|
IPM |
0.386 |
0.402 |
IBPM 3 |
0.274 |
0.405 |
|
BPM |
0.359 |
0.382 |
IBPM 5 |
0.333 |
0.402 |
|
IM |
0.240 |
0.320 |
IBPM 1 |
0.341 |
0.399 |
|
|
|
|
IBPM 4 |
0.247 |
0.383 |
All
the prepared glasses have high ionic conductivity at room temperatures (10-2 -10-4 W-1m-1).
The samples of system II. that contain always the same molar amount of glass-forming
components exhibit very close values of activation energies Ea2 characterising the
transport mechanisms at lower temperatures. The same activation energy Ea2 have also samples IPM and
BPM from system I. but containing the same concentrations of Cu2O-P2O5-MoO3
components. However, the activation energies Ea1 characterising ion transport at higher temperatures
depends on the ratio of CuI to CuBr responsible for Cu+ ion
concentrations and indicates similar role of both components in the process of
Cu+ mobile ion governing the conductivity.
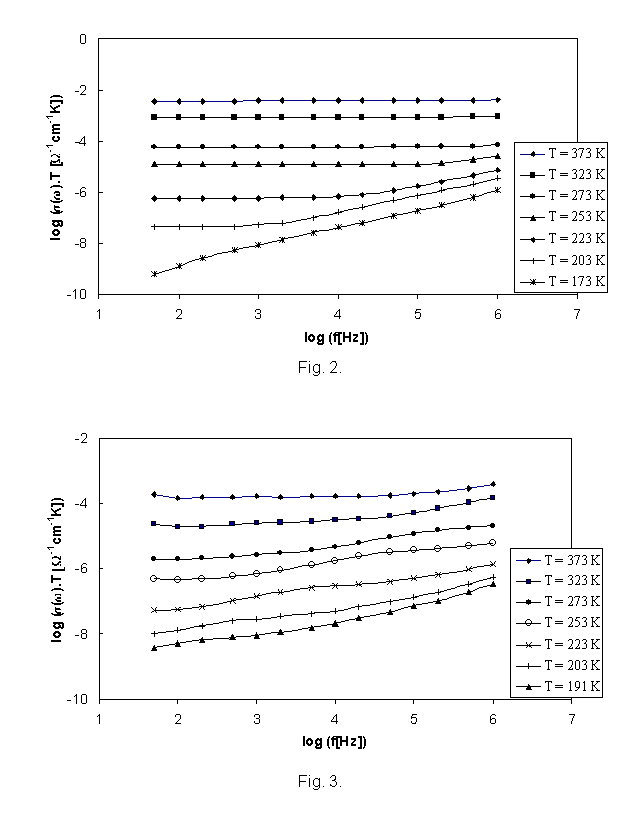
The set of
frequency dependencies of a.c. conductivity measured at various temperatures is
illustrated in Fig. 2 for glass sample IBPM 3 and in Fig. 3 for sample IPM. The
obtained a.c. conductivity measurements correspond to the complete conductivity
spectra obtained from glassy samples [7,8]. However, because of limited
frequency range, only two regimes (II and III) of [7, 8] due to hopping motion
separated by slope represented by breaks on individual curves could be
recognised, the regime II only at low temperatures yet. While the glass samples
of system II. exhibits one slope of brakes, the glass sample IPM exhibits
evidently another brakes on the a.c. conductivity spectra indicated another
transport hopping process that can be explained by slightly modified jump
relaxation model [7].
IV. Conclusion The first
experimental investigation of electrical properties both d.c. and a.c.
conductivity of ion conductive glasses in system CuI-CuBr-Cu2O-(P2O5+MoO3)
showed the important influence of chemical composition an ion transport
mechanisms and indicated more than one possible conductivity mechanism.
However,
the further investigation in wider temperature and frequency ranges of glass
samples with different compositions and comparing with the results some
different measurements should be done for better understanding of ion transport
mechanisms in investigated ion conducting glasses.
Acknowledgement This work was
partly financially supported by Grants No 1/8308/01 and No 1/9141/02 of the
Ministry of Education of the Slovak Republic
References
[1] M. D. Ingram, Phil. Mag. 60 (1989) 729.
[2] S. W. Martin, J. Amer. Ceram.
Soc. 74 (1991), 1767.
[3] T. Minami, J. Non-Cryst. Solids 119 (1990) 95.
[4] P. Znášik and M. Jamnický, Solid
St. Ionics 95 (1997) 207.
[5] Ch. Lin and C.A. Angel, Solid
St. Ionics 13 (1984) 105-
[6] T. Minami and N. Machida, Mater.
Chem. Phys. 23 (1989) 63.
[7] K. Funke, Sol. State Ionics 94 94 (1997) 27.
[8] K. Funke, B. Roling, M. Lange,
Sol. State Ionics 105 (1998) 195.