Correlation between Electrical and Acoustical Properties of Ion Conductive Glasses
I. Jamnický a, P. Bury a, M. Jamnický b and P. Hockicko a
a Department of Physics, Žilina University, 010 26 Žilina, Slovakia
b Department of Ceramic, Glass and Cement, Slovak Technical University, 812 37 Bratislava, Slovakia
introduction
In recent years, the technological interest in fast ionic conductivity in solid materials is increased for various solid state electrochemical devices. Apart from some crystalline materials, the high ionic conductivity at room temperature has been observed in some ion conducting glasses. Glass materials are particularly interesting for application because of their easy preparation, their stability and the large available composition ranges.
Ionic conductive glasses have common structural characteristic, that includes a highly ordered, immobile framework complemented by a highly disordered interstitial sublattice in which carriers are randomly distributed and in which the number of equivalent sites is greater than the number of available ions to fill them. These low potential sites comprising the carrier sublattice must be sufficiently interlined to provide continuous transport paths necessary for optimal movement of ions [1].
The most important criterion which should be meet the fast ionic conductive glasses is high ionic conductivity at room temperature. The highest ionic conductivity (s25~10-2W-1) were obtained in Ag+ containing glasses. Because of a shortage of silver it is necessary to find new chemical composition of glasses, with conductivity comparable to the Ag+ containing glasses. Here is a good opportunity for glasses containing Cu+ conductive ions that have similar electronic configuration and smaller ionic radii in comparison with Ag+ ion. From a theoretical point of view, this is a good chance to achieve conductivity comparable with the best Ag+ conductive glasses [2].
The conductivity of glasses is affected not only by the type of conductive ions, but also strongly depends on „glass forming“ oxide. The glass formation of many systems containing Cu+ conductive ions was investigated [3], but glasses were prepared only in systems containing either P2O5 or M0O3 as a „glass forming oxide“. It has been found that the mixing of two glass formers yields glasses with higher electrical conductivity. Such glasses were also prepared in systems with mixed „glass forming“ oxides P2O5-MoO3 [3,4].
In the present contribution the ion conducting glass of the system 0,3CuI – 0,4375Cu2O – 0,0875P2O5 – 0,1750MoO3 was chased for the experimental investigation, its electrical and acoustical properties were studied and some relations between electrical conductivity and acoustical attenuation is discussed.
EXPERIMENTAL AND RESULTS
The preparation procedure of glasses in the system CuI – Cu2O – P2O5 – MoO3 has been already described [5]. The glasses investigated here were prepared in a similar manner to the previously described method. The glass melt was rapidly quenched by its pressing between two brass plates to a final thickness of » 1,5 mm. The resulting disc was kept between the plates until the temperature decreased to room temperature. The chemical compositions of glasses are given in previous part.
The samples for both the electrical conductivity and acoustical attenuation measurements were cylindrical in shape (area » 1 cm2, thickness » 1,5 mm). Gold electrodes were sputtered onto the sample surfaces. The frequency and temperature dependencies of electrical conductivity were measured in the frequency range from 50 Hz to 1 MHz using FLUKE PM 6306 impedance analyser from temperature up to ~ 100°C. The measured complex impedance allowed us to obtain the bulk d.c. and a.c. conductivity of glass samples by means of the usual impedance analysis. All of the temperature dependencies of glass conductivity fitted the equation
s T = s0 exp (-Ea / kT) ,
where Ea is the activation energy, k is the Boltzman constant and T is the thermodynamic temperature.
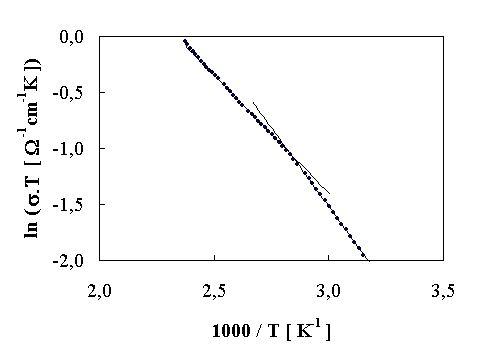
FIGURE 1. Arrhenius plot of d.c. conducitivity
The d.c. electrical conductivity against the temperature is plotted in Fig. 1. The Arrhenius plots enables both the Ea and pre-exponential factor calculation. The temperature dependence of conductivity (Fig.1) indicates two conductivity mechanisms with activation energy 0,18 eV or/and 0,24 eV, respectively. The frequency dependence of a.c. conductivity confirmed the supposition about not very significant influence of hopping processes up to 1 MHz [6]. The temperature dependence of a.c. conductivity is showed in Fig. 2.
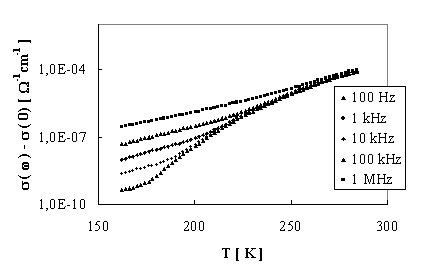
FIGURE 2. Temperature dependence of a.c. conductivity
The acoustical attenuation was measured using MATEC attenuation comparator for longitudinal acoustic wave of frequency 13 MHz generated by quartz transducer. The quartz buffer was used to separate the signal from quite short sample. The temperature dependence of acoustic wave is showed in Fig. 3.
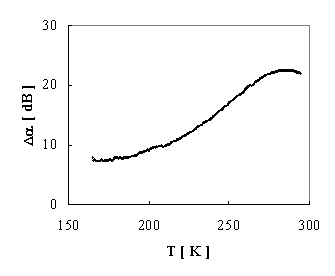
FIGURE 3. Temperature dependence of acoustic wave attenuation
The first experimental investigation of electrical and acoustical properties of ion conductive glasses in system CuI – Cu2O – (P2O5 – MoO3) indicate the important fact that the same mechanism can probably influence the electrical and acoustical losses in the ion conducting glasses and indicated also more than one possible conductivity mechanism. Relaxation character of measured acoustical (a) and electrical (s) parameters is probably due to ion hopping process. However, the further investigations in wider temperature and frequency ranges of samples in wider scale of compositions and combined with some different measurements should he done for better understanding of ion conductivity mechanism.
Acknowledgements
This work partly financially supported by Grants No 1/8308/01 and No 1/830801 of the Ministry of Education of the Slovak Republic.
References
1. D.P. Button, L. S. Mason, H. L. Tuller and D.R. Uhlmann, Solid State Ionics 9/10 585 (1983)
2. T. Minami, J. Non-Cryst. Solids 73, 273 (1985)
3. N. Machida, M. Chusho, T. Minami, J. Non-Cryst. Solids 101, 70 (1988)
4. B.V.R. Chowdari, K.L. Tan, W.T. Chia, R. Gopalakrishnan, J. Non-Cryst. Solids 119, 95 (1990)
5. P. Znášik, M. Jamnický, Solid State Ionics 95, 207 (1997)
6. K. Funke, B. Roling, M. Lange, Solid State Ionics 105, 195 (1998)