Introduction A considerable interest is given in
experimental study of glassy materials with the fast ion transport because they play an important role in a
number of modern electrochemical devices, such as solid-state batteries,
electrochronic displays, and sensors as well as for fundamental interest in
their ion transport mechanisms [1,2]. The ion conductive glasses have several
advantages comparing with crystalline materials because of their easy
preparation, their stability and the large available composition ranges.
It
is known that the investigation of conductivity spectra of ionic glasses can
reflects the basic features of the relaxation and transport mechanisms of the
mobile ions and the high ion conductivity at room temperature is the most
important criterion with should be meet the fast ion conductive glasses [3,4].
However, the transport mechanisms can be investigated also by acoustic methods,
that can have some advantages comparing to electrical ones as the high
sensitivity, absence of contact phenomena and so on [4].
Here
is a good opportunity for glasses containing Cu+ conductive ions
that have similar electronic configuration and smaller ionic radii in
comparison with Ag+-ion conducting glasses in various glass-forming
systems, Cu+-ion conducting glasses are only known in very limited
glass-forming systems. Phosphate glasses containing Cu+ conducting
ions are good ionic conductors with room temperature conductivity of the order
10-3W-1cm-1 [6,7]. The highest conductivity
has been recorded in systems containing large more fractions of cuprous
halides, such as CuI or CuBr. Moreover, if two different kinds of halide anions
are mixed into cation conducting glasses [8], a positive deviation of the
electrical conductivity from the additivity rule can be observed (mixed anion
effect). In this contribution we present some acoustical and electrical
properties of glasses prepared in the systems CuI-CuBr-Cu2O-MmOn
where MmOn is P2O5 and/or MoO3.
The main purpose of the contribution is to investigate ion transport mechanisms
and to determine the relation between acoustical and electrical properties for
various glass composition.
Experimental Procedure The preparation of glasses in the
system CuI-CuBr-Cu2O-(P2O5+MoO3)
from commercial reagents (Fluka) represented the procedure already described
[8]. Batches of 15 g were melted under a dry argon atmosphere to avoid the
oxidation of Cu+ during melting and mixed in appropriate portion in
silica ampoule at 933 K for 90 min. The glass melts were rapidly quenched
by pressing discs of 20 mm in diameter were kept between the plates until their
temperature decreased to room temperature. Losses in weight during melting were
< 1%. To check th reproducibility of the
results, all glasses were prepared three times. Two systems of glasses were
orriginally prepared to investigate both the role of glass-forming system and
the role of cuprous halides produced Cu+ ions keeping their ratio 60
mol. % to 40 mol.% [9]. However, for the acoustical attenuation measurements
only three samples were chosen (d»2 mm), the compositions of which is
summarized in Table 1.
The
samples for acoustical attenuation and electrical conductivity measurements
were cylindrical in shape (area » 1 cm2, thickness » 2 mm). Gold electrodes were sputtered onto the
sample surfaces for electrical investigation. The frequency and temperature
dependencies of electrical conductivity were measured (d.c. and a.c. in the
frequency range from 50 Hz up to 1 MHz) using FLUKE PM 6306 impedance analyser
and in the temperature range of 140-380 K. The measured complex impedance
allowed us to obtain the bulk d.c. and a.c. conductivity of glass samples by
means of the usual impedance analysis.
The
acoustical attenuation was measured using MATEC attenuation comparator for
longitudinal acoustic wave of frequency 13 MHz generated by quartz transducer.
The quartz buffer was used to separate the signal from quite short sample.
Table 1
Starting glass compositions (in mol.%)
|
Glass sample |
Composition
(in mol.%) |
||||
|
CuI |
CuBr |
Cu2O |
P2O5 |
MoO3 |
|
|
IPM |
25.000 |
- |
46.875 |
9.375 |
18.750 |
|
BPM |
- |
25.000 |
46.875 |
9.375 |
18.750 |
|
IBPM 5 |
15.625 |
9.375 |
46.875 |
9.375 |
18.750 |
Results The measurements of temperature
dependence of acoustic attenuation (Fig. 1) indicate one broad attenuation
maxima in all investigated samples, in which
we can distinguish two separated peaks with the different positions for every
sample.
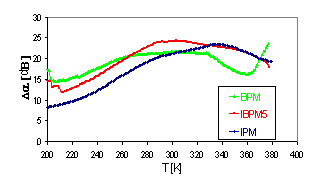
Fig.1 Temperature dependence
of acoustic attenuation
Except the broad maximum with two peaks the sample
BPM exhibits also the rapid attenuation increase at higher temperature
indicated another maximum. The measured complex impedance allowed to obtain the
bulk d.c. and a.c. conductivity of glass samples given temperature range as all
of the temperature dependence of d.c. glass conductivity can be fitted by the
equation
s = s0exp(-Ea/kT), (1)
where Ea is the activation energy,
k is the Boltzman constant and T is the absolute temperature, the
temperature dependencies of d.c. conductivity indicate two transport mechanisms
with activation energies Ea1, and Ea2 for higher and
lower temperatures, respectively. Because the pre-exponential factor s0 is a function of temperature the factor sT is used in Arrhenius plots of d.c. conductivity.
Activation energies calculated from Arrhenius plots of d.c. conductivity for
all glass samples are summarised in Table 2.
Table 2 Summary of activation energies
calculated from Arrhenius plots of d.c. conductivity
|
Glass sample |
Ea1 (eV) |
Ea2 (eV) |
|
IPM |
0.386 |
0.402 |
|
BPM |
0.359 |
0.382 |
|
IBPM5 |
0.333 |
0.402 |
All
prepared glasses have high ionic conductivity at room temperature (10-2 -
10-4W-1m-1). The samples containing the same
molar amount of glass-forming components exhibit very close values of
activation energies Ea2 characterising the transport mechanisms at
lower temperatures. However, the activation energies Ea1
characterising ion transport at higher temperatures depends on the ratio of CuI
to CuBr responsible for Cu+ ion concentrations and indicates similar
role of both components in the process of Cu+ mobile ion governing
the conductivity. Two peaks at the broad maxima of acoustical attenuation
spectra indicate two possible transport mechanisms with very close activation
energies to those determined in d.c. electric measurement.
The
set of frequency dependencies of a.c. conductivity measured at various
temperatures is illustrated in Fig.2 for glass sample IBPM5 and in Fig.3 for
sample IPM.
The obtained a.c. conductivity measurements
correspond to the complete conductivity spectra obtained from glassy samples
[3,10]. However, because of limited frequency rage, only two regimes (II and
III) of [3,10] due to hopping motion separated by slope represented by breaks
on individual curves could be recognised, the regime II only at low
temperatures yet.
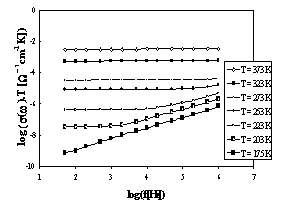
Fig.2 The frequency dependence
of a.c. conductivity at various temperatures for sample IBPM 5
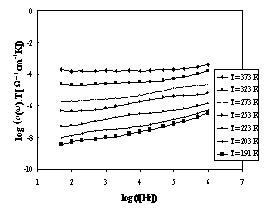
Fig.3 The frequency dependence of a.c.
conductivity at various temperatures for sample IPM
While the glass sample IBPM5 exhibits one slope
of brakes, the glass sample IPM exhibits evidently another brakes on the a.c.
conductivity spectra indicated another transport hopping process that can be
explained by slightly modified jump relaxation model [3].
The
study of mechanical losses of mixed cation glasses [11] exhibits an Arrhenius -
type relation between peak temperature and applied frequency
n = n0exp(-Ea/kTpeak) , (2)
where the values for preexponential factor n0 are always of order of 1014Hz.
Because the acoustic attenuation measurement were performed at the same
frequency n, the activation energy Ea
is proportional to the peak temperature Tpeak. The acoustical
spectra show large mechanical loss peaks with activation energies very close to
those of d.c. conductivities (Ea1 = 0,35 eV, Ea2 = 0,42
eV for sample BPM, Ea1 = 0,38 eV, Ea2 = 0,43 eV for
sample IPM). The attenuation spectra can be then explained by the assumption
that temperature peaks are caused by the diffusion processes of various kinds
of ions.
Conclusion The experimental investigation of acoustical and electrical
properties of ion conductive glasses in system CuI-CuBr-Cu2O-(P2O5+MoO3)
showed the important influence of chemical composition and ion transport
mechanisms and indicated more than one transport mechanism. The fact that the
activation energies determined from both electrical conductivity measurement
and acoustical attenuation spectra have very similar values proved that the
same mechanisms can influence electrical and acoustical losses in ion
conductive glasses.
However,
the further investigation in wider temperature and frequency ranges of glass
samples with different compositions and comparing with the results of some
different measurements should be done for better understanding of ion transport
mechanisms in investigated ion conducting glasses.
Acknowledgement The authors would like to thank Mr.
F. Černobila for technical assistance. This work was partly financially
supported by Grants No 1/8308/01 and No 1/914/02 of the Ministry of Education
of the Slovak Republic.
References
[1]
M.D.Ingram, Phil. Mag. 60 (1998)
729.
[2] S.W.Martin, J.Amer. Ceram.
Soc. 74 (1991), 1767.
[3]
K. Funke, Sol. State Ionics 94
(1997) 27.
[4] E.V. Charnaya,
B.F.Borisov, A.A.Kuleshov, Proc. World Congress on Ultrasonics, Berlin 1995, p.
483.
[5]
T. Minami, J. Non-Cryst. Solids 119
(1990) 95.
[6] P. Znášik and M. Jamnický,
Solid St. Ionics 95 (1997) 207.
[7]
Ch. Lin and C.A.Angel, Solid St. Ionics 13
(1984) 105.
[8] T. Minami and N. Machida,
Mater. chem. Phys. 23 (1989) 63.
[9] P. Bury, P. Hockicko, M.
Jamnický and I. Jamnický, Proc. 8th Int. Worshop on Appl. Phys. of
Cond. Matter, Jasná 2002, p. 145.
[10] K. Funke, B. Roling, M. Lange, Sol. State Ionics 105
(1998) 195.
[11] B. Roling, A. Happe, M.D.
Ingram and K. Funke, J. Phys. Chem. B 103
(1999) 4122.